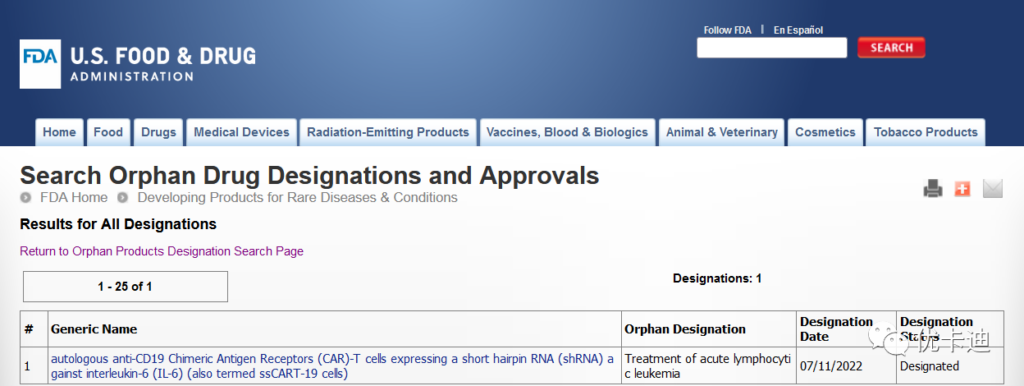
Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd. (hereinafter referred to as “Unicar”) announced that its independently developed product sscart-19 cell injection was granted orphan drug qualification (odd) by the U.S. Food and Drug Administration (FDA) on July 11, 2022 for the treatment of acute B-lymphocyte leukemia (B-ALL). This means that sscart-19 cell injection will enjoy preferential approval, market monopoly, tax relief, R & D support and other policy support for clinical trials in the United States.
Dr. Yu Lei, chairman and chief scientist of Unicar, said: “sscart-19 injection is the world’s first new generation of car-t innovative technology product developed based on Unicar original smart gene regulation and enabling technology platform. This time, FDA awarded the title of sscart-19 orphan drug, which fully demonstrates FDA’s recognition of the safety and effectiveness of this therapy, and also marks another important step forward in the internationalization process of Unicar.”
In September 2020, Unicar successfully obtained the clinical trial license of sscart-19 cell injection, the world’s first safe car-t product. This product can be used for the treatment of central nervous system leukemia (leukaemia), a contraindication of car-t, and is a major milestone in the treatment of blood tumors with car-t therapy.