Chimeric antigen receptor T cell (CAR-T) therapy is one of the most promising treatment techniques for recurrent and refractory (R/R) acute B-ALL. Among them, CD19 CAR-T therapy is the most commonly used and has a relatively stable effect. It can induce a higher complete remission (CR) rate in patients with poor prognosis and few treatment options, and the incidence of grade 3 and 4 adverse events is relatively low.
But at this stage, how to apply existing CAR-T therapy more suitable for R/R B-ALL patients is a controversial point. Is it CD19 single target application or CD19/CD22 dual target application? A recent study published in the Blood Cancer Journal explored this issue.
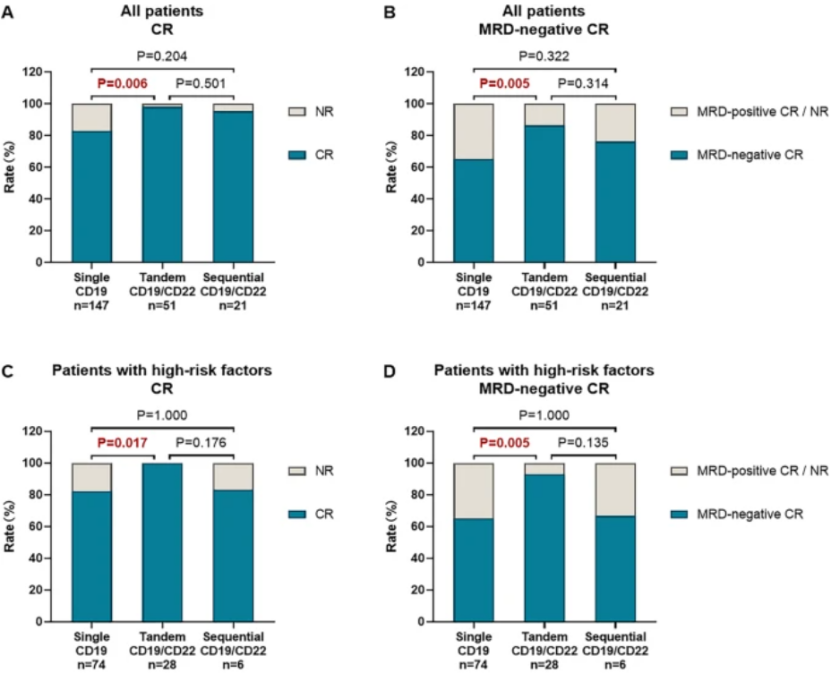
The research results showed that the CR rate of patients in the serial CD19/CD22 CAR-T therapy group was significantly higher than that in the single CD19 CAR-T therapy group (98.0% vs 83.0%, P=0.006), and was similar to that in the sequential CD19/CD22 CAR-T therapy group (98.0% vs 95.2%, P=0.501) (Figure 2A).
The serial CD19/CD22 CAR-T therapy group was one of the significant favorable factors in the multivariate analysis of CR rate. The negative CR rate of minimal residual disease (MRD) in the single CD19 CAR-T therapy group was 65.3%, while in the serial CD19/CD22 CAR-T therapy group and sequential CD19/CD22 CAR-T therapy group, it was 86.3% and 76.2%, respectively (Figure 2B). The high-risk patients with MRD negative CR rate in the serial CD19/CD22 CAR-T therapy group were significantly higher than those in the single CD19 CAR-T therapy group (92.9% vs 64.9%, P=0.005) (Figure 2D).
Conduct subgroup analysis on the dual target CAR-T treatment method. Compared with the single CD19 CAR-T therapy group, patients in the serial CD19/CD22 CAR-T therapy group had higher CR rates with the following characteristics: no history of allogeneic hematopoietic stem cell transplantation (Allo HSCT) (84.5% vs 100.0%, P=0.004), no extramedullary lesions (EMD) (88.0% vs 100.0%, P=0.013), high tumor burden (78.2% vs 100.0%, P=0.014), and no complex karyotypes (83.0% vs 97.8%, P=0.011).
The CR rate of high-risk patients in the serial CD19/CD22 CAR-T therapy group was higher than that in the single CD19 CAR-T therapy group (100.0% vs 82.4%, P=0.017) and the sequential CD19/CD22 CAR-T therapy group (100.0% vs 83.3%, P=0.176) (Figure 2C)
From this, it can be seen that serial CD19/CD22 CAR-T therapy is superior to single CD19 CAR-T therapy and can generate better treatment response in R/R B-ALL patients.
Research shows that the incidence of adverse events is similar among the three groups of patients. For example, 168 out of 219 patients (76.7%) developed cytokine release syndrome (CRS), of which 22.4% were severe grade 3-4 CRS. 25.9% of patients in the single CD19 CAR-T therapy group developed severe CRS, 13.7% in the serial CD19/CD22 CAR-T therapy group, and 19.0% in the sequential CD19/CD22 CAR-T therapy group (P=0.196).
After CAR-T therapy, some R/R B-ALL patients who achieved CR were bridged for Allo HSCT, which significantly improved their clinical outcomes. Patients who received Allo HSCT had a median leukemia free survival (LFS) better than those who did not receive Allo HSCT (less than 10.9 months). Multivariate analysis of CR patients showed that low recurrence rate, low tumor burden, negative MRD CR, and bridging transplantation were independently associated with better LFS rates.
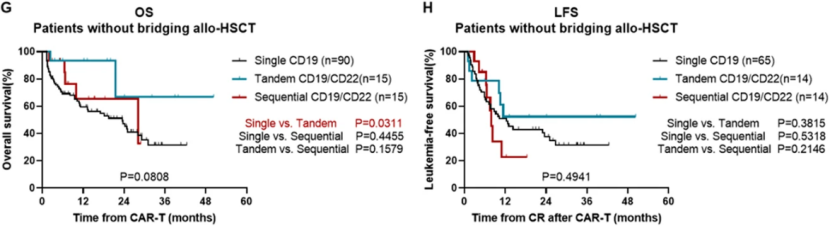
Among patients who did not receive Allo HSCT, the median overall survival (OS) of 15 patients in the serial CD19/CD22 CAR-T therapy group was significantly longer than that in the single CD19 CAR-T therapy group (not reaching 23.5 months, P=0.0311) (Figure 3G). The OS of patients without high-risk factors in the serial CD19/CD22 CAR-T therapy group was significantly better than that of the single CD19 CAR-T therapy group (3-year OS: 75.0% vs 21.2%, P=0.0257).
The median LFS was 12.4 months in the single CD19 CAR-T therapy group, but not in the serial CD19/CD22 CAR-T therapy group. The median LFS was 7.9 months in the sequential CD19/CD22 CAR-T therapy group, and there was no difference in survival between the single CD19 CAR-T therapy group and the sequential CD19/CD22 CAR-T therapy group (P=0.4941) (Figure 3H).
The dual target CAR-T therapy of serial CD19/CD22 achieved better response than single CD19 CAR-T therapy in the treatment of R/R B-ALL, while the efficacy of sequential CD19/CD22 CAR-T therapy was similar to that of serial CD19/CD22 CAR-T therapy. Serial CD19/CD22 CAR-T therapy may be more suitable for treating R/R B-ALL patients.