China CAR-T Cell
Help patients with CAR-T Matters!
Help patients with CAR-T Matters!
CAR T-cell therapy may have some severe side effects, and that’s why we monitor patients very closely. We ask that patients stay in the hospital for 30-60 days after the infution because we are going to be seeing them very frequently.
One of the major side effects is something called Cytokine release syndrome (CRS). It occurs because a large number of CAR-T cells are infused into the body, resulting in the activation of the immune system and the release of a large number of inflammatory cytokines, resulting in serious adverse reactions in the human body. This presents like the patients have an infection. They can develop high fevers, a high heart rate and low blood pressure. Some patients will need to go to the intensive care unit for a higher level of care. That is why we monitor them very carefully in the hospital for the first two to three weeks after they receive the CAR T-cell therapy. Anyway, our physicians here are very experienced. We have formed a series of measures to keep it under control.
The cause of neurotoxicity is not very clear, which may be related to CRS. Clinical manifestations include headache, delirium, partial loss of language ability, slow response, seizures, coma, and even death caused by brain edema.
Allergic reactions are also predictable adverse reactions. It is mainly rash. Other reactions cannot be distinguished from CRS. Symptoms such as dyspnea, edema and decreased blood pressure will be attributed to CRS reaction.
Whether at home or abroad, the safety of CAR-T therapy has always been the focus of attention. At present, side effects of CAR-T therapy are inevitable. CAR-T therapy is not completely accurate to only target tumor cells, but has no effect on normal human cells.
CAR-T cells need to be monitored for 15 days to 4 weeks after infusion. The first 15 days need to be hospitalized to closely monitor side effects. Within 4 weeks after discharge, the patients should not be too far away from the hospital to facilitate the timely treatment of side effects in the hospital.
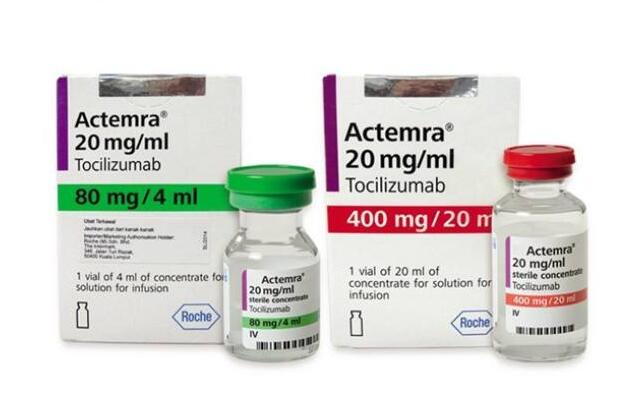
Through the efforts in recent years, the current treatment institutions have formed a set of plans to deal with the side effects of CAR-T therapy, including effective drugs/medicines for the treatment of cytokine release syndrome: Anti-IL-6 receptor antibody tocilizumab to reduce the level of IL-6 in the blood, and the use of hormones can well control its side effects (nccn2018 immunotherapy guidelines)
By improving clinical predictability and identifying high-risk factors of side effects, the probability of side effects can be reduced. For example, by adding a chemotherapy scheme to reduce the tumor load in the pre-chemotherapy before the CAR-T cells are infused into the patient, the tumor load in the patient can be reduced and the side effects of treatment can be reduced.
The decrease of whole blood cells during CAR-T cell treatment is very common. For patients with infection, it is very likely to aggravate the infection during the treatment. If the patient’s infection is not controlled, CAR-T cells should not be reinfusion temporarily, and should be reused after the infection is effectively controlled. At the same time, for patients with pancytopenia during treatment, necessary antibiotic treatment can effectively prevent the occurrence of infection. For patients with long-term follow-up after CAR-T treatment, the reduction of the number of B cells and immunoglobulins is also very common. It is suggested that monthly immunoglobulin detection and c-globulin infusion can effectively avoid dangerous infection caused by hypoglobulinemia.
Interestingly enough, what we saw was that developing the syndrome actually correlated with responding well to the treatment. So it is a sign that the patient is responding well to the CAR T-cell therapy.